Introduction

Welcome to the Liddle Research Group web pages!
We are based at the School of Chemistry, University of Manchester and are interested in all aspects of organometallic chemistry across the periodic table. Our research has a particular emphasis on the chemistry of lanthanide (we include group 3 metals in this definition) and actinide (principally uranium and thorium but also neptunium and plutonium) complexes which challenge preconceived ideas.
Specific topics which we are currently investigating include:
- uranium-metal(loidal) bonds.
- f-block carbenes.
- uranium-ligand multiple bonding.
- f-block single molecule magnets.
- small molecule activation.
Our work has received generous support from a wide range of sources including the Royal Society, EPSRC, ERC, Marie Curie IIF, COST, NSCCS, Nuffield Foundation, University of Nottingham, University of Manchester, and the National Nuclear Laboratories, and this has resulted in ~150 publications.
We collaborate with research groups based within Manchester, the UK, and internationally, and our work has been regularly highlighted in Chemical Engineering News, Chemistry World, the Materials Research Society, Chemistry in Australia, and Nature Chemistry.
We are also active in communication of science to the public and have been part of the award-winning Periodic Videos project which can be found on YouTube.
Our research is carried out in modern, well equipped labs, where we routinely employ Schlenk and Glovebox methods, and in an integrated approach we use a wide variety of structural, spectroscopic, magnetic, and computational techniques to comprehensively characterise new compounds and reactions.
If you are interested in joining our group, and/or are interested in our science and what excites us, then some highlights are illustrated to the right and further information and contact details can be found by following the links shown to the left. If you require any further information about our work please feel free to contact us directly.
Highlights
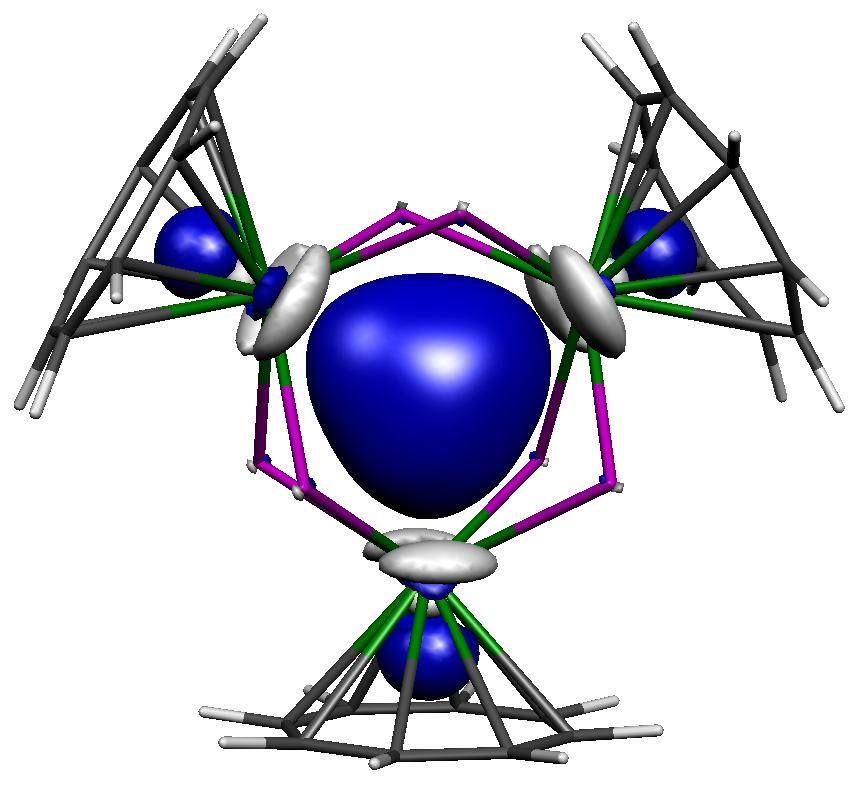
Isolable actinide-actinide bonding, and not what you would expect it to be! See: "A crystalline tri-thorium cluster with σ-aromatic metal-metal bonding" Nature, 2021, 598, 72-75.
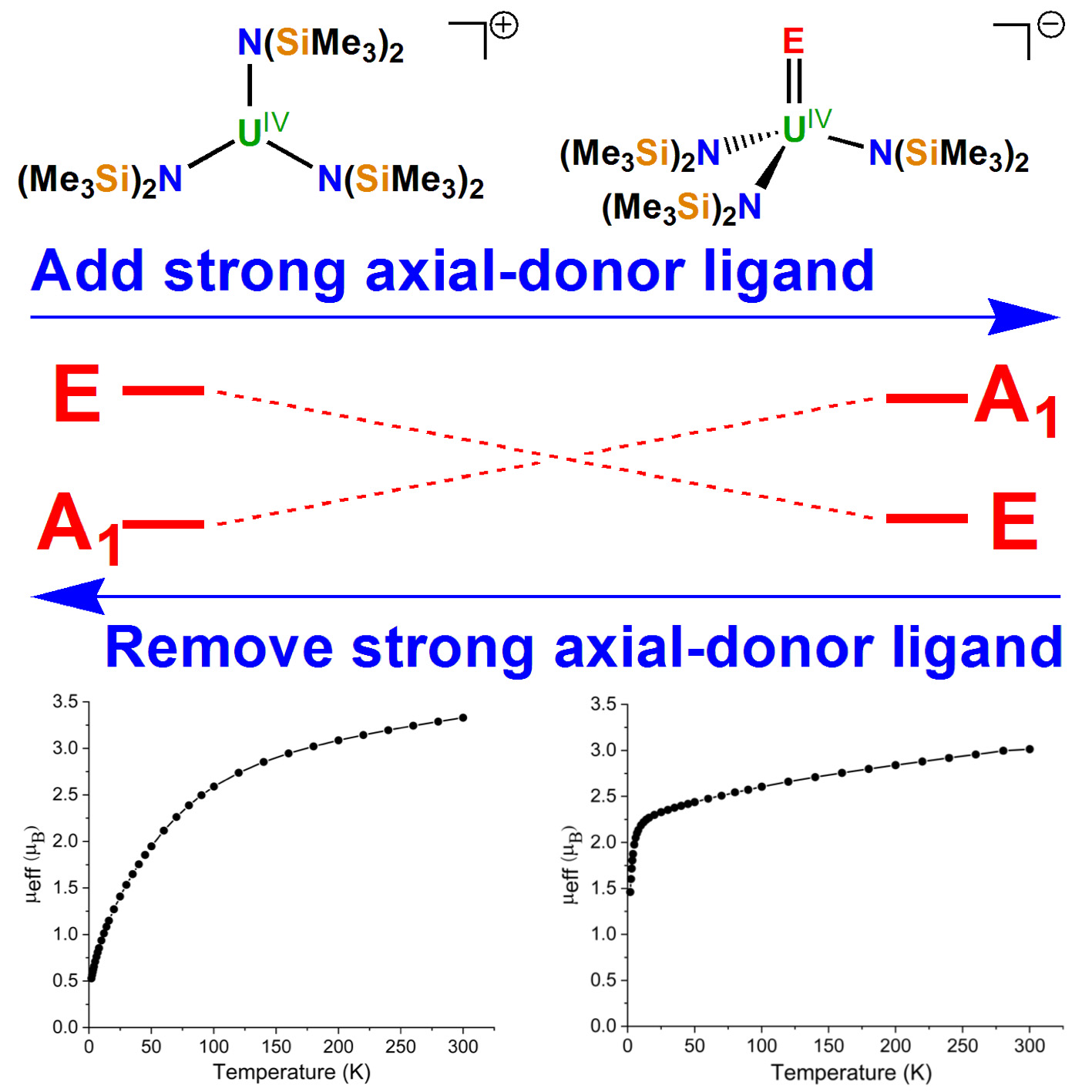
Rationalising the increasing number of anomalous low-temperature magnetic moments of uranium(IV) complexes. See: "Anomalous Magnetism of Uranium(IV)-Oxo and -Imido Complexes Reveals Unusual Doubly Degenerate Electronic Ground States" Chem., 2021, 7, 1666-1680.