Karel Dorey Laboratory

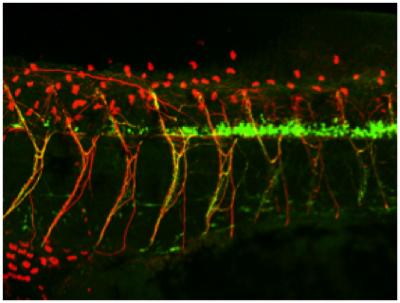
Spinal cord development and regeneration
In my laboratory, we aim to understand the molecular and cellular mechanisms regulating spinal cord development and regeneration. Mammals, including humans, have a poor ability to repair their spinal cord upon injury. About 27 million people worldwide suffer long-term disability following spinal cord injury (SCI). Depending on the severity of the injury it can cause irreversible damage, which can lead to the loss of motor and sensory function posterior to the site of the damage. This has far reaching consequences for the life of the individual as they face lifelong confinement in wheelchair, dependency on medical care and the risk of premature death. Therefore, there is a high requirement for improving regenerative capabilities of the spinal cord after injury.
However, some vertebrates can regenerate their spinal cord. This is the case of zebrafish, the newt Axolotl and the tadpoles of the amphibian Xenopus. The genome of Xenopus tropicalis has been sequenced and shows striking similarities with the human genome, meaning that findings from Xenopus provide insight into many human conditions and diseases. Furthermore, Xenopus sits at an interesting juncture in term of regeneration. During its tadpole stages, Xenopus can regenerate most tissues including its spinal cord however this ability is lost after metamorphosis, allowing comparative studies within one species.
Recently, we have used state-of-the-art technology to start unravelling the mechanism underlying spinal cord regeneration. In particular, we have developed new methodologies to characterise the changes in gene expression in the regenerating spinal cord using bulk and single cell RNA sequencing. Using functional studies such as generating Crispr/Cas9 – mediated knockout, we have uncovered a new role for neuronal differentiation during appendage regeneration.
We are also interested in the mechanisms regulating spinal cord development. Our scRNAseq dataset revealed that the organisation of Xenopus tadpoles spinal cord resemble that of mammalian embryos. Furthermore, we are studying the role of structural proteins such as Lrig2 in the differentiation and function of motoneurons.


