|
|
PERVAPORATION |
MENU
|
PERVAP- ORATION |
||
|
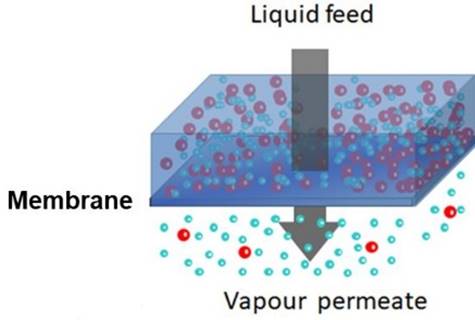
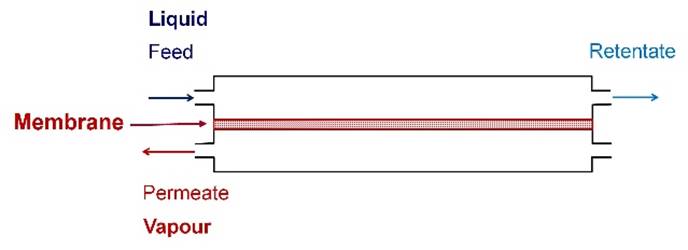
Pervaporation is a membrane process that
combines permeation and evaporation. The feed is a liquid mixture. A vacuum or sweep gas is used to remove permeate
as a vapour. |
|
|
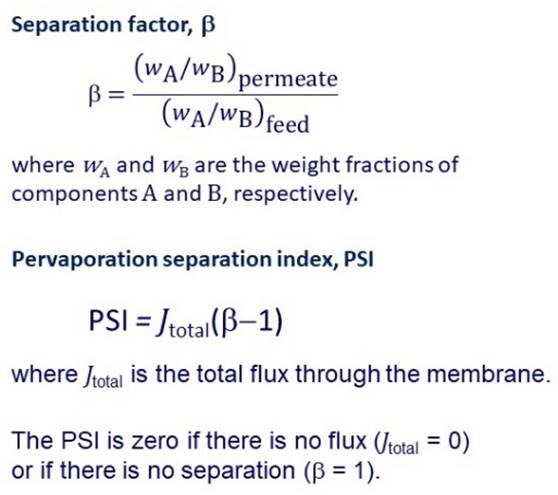
The ability of a membrane to concentrate up a component of a
mixture may be expressed in terms of a separation factor. We need to achieve both a good
separation factor and a high flux through the membrane. The overall performance of a
membrane may be expressed in terms of a pervaporation separation index. |
|
|
|
HYDROPHILIC
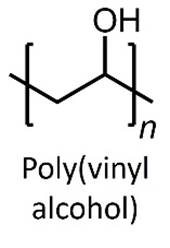
PERVAPORATION Hydrophilic pervaporation
membranes are used for the dehydration of (removal of water from) organic
solvents. Hydrophilic pervaporation membranes
are commonly based on polymers such as poly(vinyl
alcohol). Inorganic pervaporation
membranes are also available. |
|
ORGANOPHILIC PERVAPORATION Organophilic pervaporation membranes are used for the separation
of organic/organic mixtures, and for the removal of organic compounds from
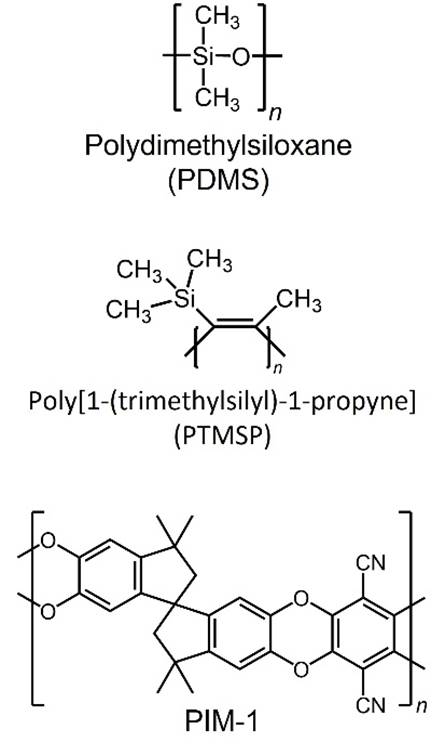
aqueous solution. Organophilic membrane polymers include polydimethylsiloxane
(PDMS), poly[1-(trimethylsilyl)-1-propyne]
(PTMSP) and polymers of intrinsic microporosity such as PIM-1. |
|
|
PIM-based membranes are being investigated
for applications such as the recovery of biobutanol
from fermentation processes. |
|
|
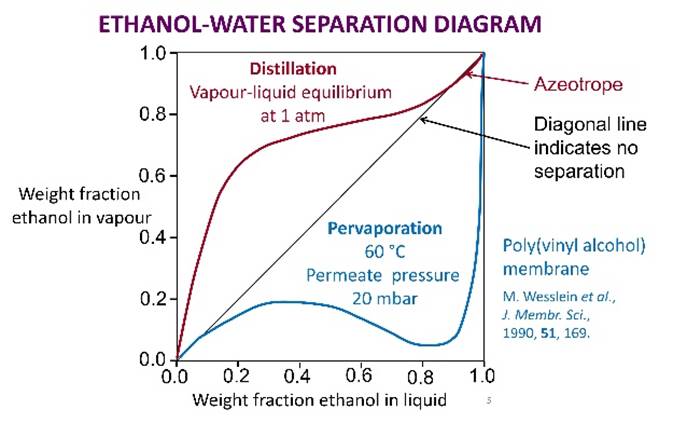
AZEOTROPES Some liquid mixtures cannot be separated by straightforward
distillation because they form azeotropes.
For an azeotrope, the vapour and liquid compositions are identical. |
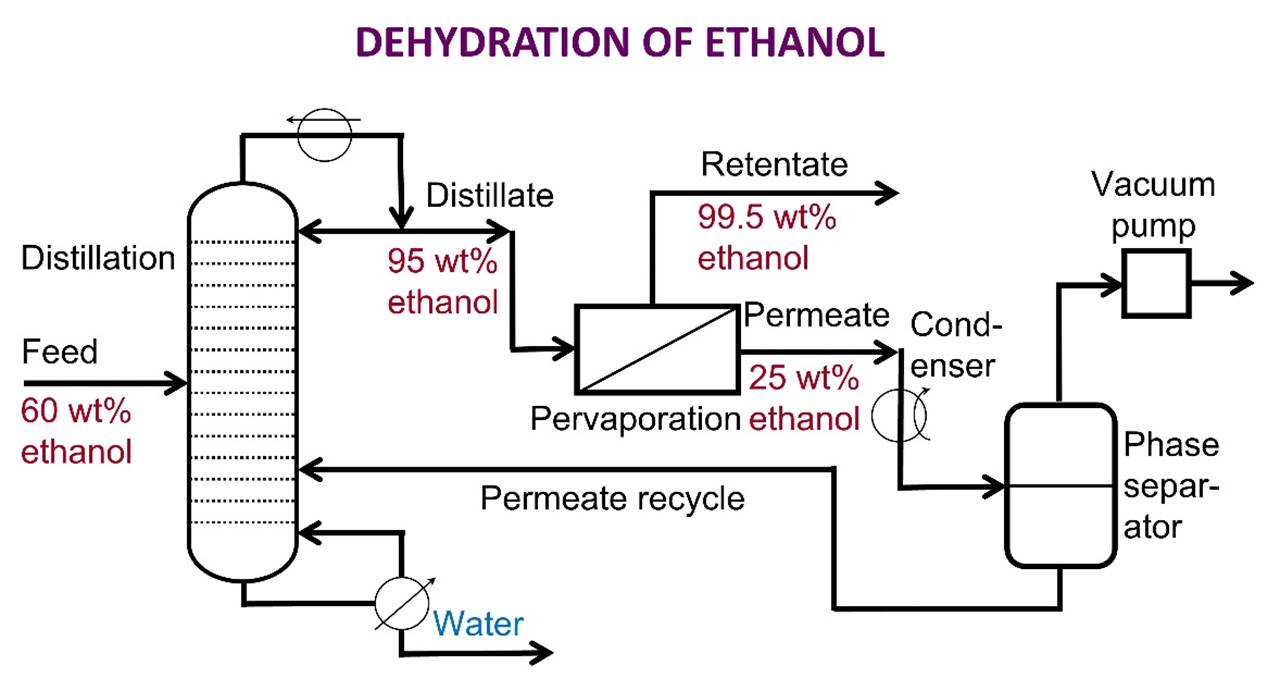
ETHANOL/WATER SEPARATION A mixture of ethanol
and water forms an azeotrope
that is 95.6 wt.% ethanol. Higher concentrations of ethanol cannot be
obtained by straightforward distillation, but they can with a hybrid process of distillation followed by pervaporation. |
|
|
|
|
To compare with simple distillation, a useful plot is of vapour composition against liquid composition, either in terms of mole fraction or
of weight fraction. A diagonal line in such a plot indicates where vapour and liquid compositions are identical (no
separation occurs). |
|
|
|
|