|
|
MEMBRANE APPLICATIONS |
MENU
|
MEMBRANE APPLICATIONS |
||
|
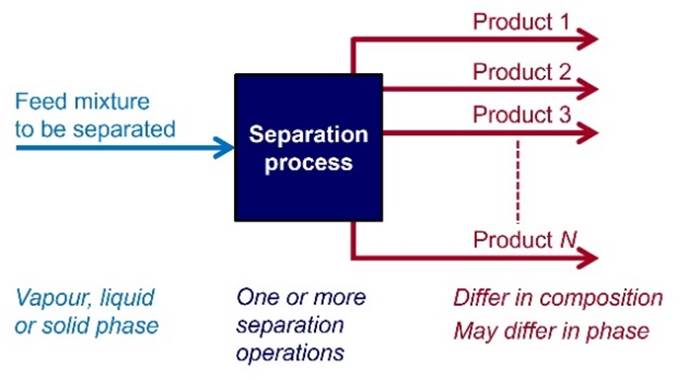
SEPARATIONS Almost every element or compound is found
naturally in an impure state, as a mixture that needs to be separated into its
individual components. Common industrial separation processes include
distillation, crystallization, liquid-liquid extraction, absorption,
adsorption and membrane processes. |
|
|
10-15% of the world’s energy consumption is used
in separations. Some separation processes, such as distillation, are very energy
intensive. New techniques, such as membrane processes, can
reduce energy use, lessen environmental impact, and provide more sustainable
ways to obtain what we need. |
|
|
|
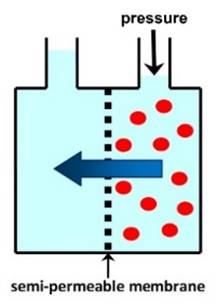
DESALINATION Membranes may be used to remove sodium chloride
and other ions from seawater (salt concentration 3-4%) or brackish water
(salt concentration <3%), to provide clean water for drinking or other
uses. The main technique used for desalination of
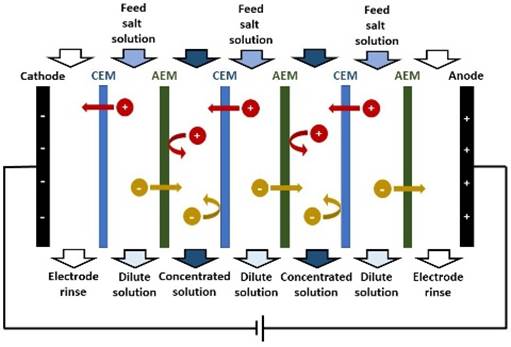
seawater is REVERSE OSMOSIS. Other membrane processes used for desalination include
ELECTRODIALYSIS. |
|
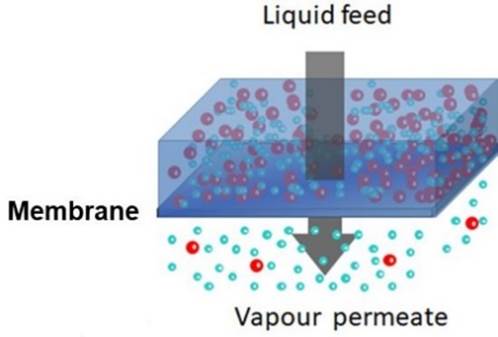
DEHYDRATION Some mixtures are particularly
difficult to separate by conventional methods. For example, you cannot obtain
pure ethanol by simple distillation of a mixture with water, because an
azeotrope is formed that is 95.6 wt.% ethanol. PERVAPORATION may be used to
remove water and achieve purer ethanol. |
|
|
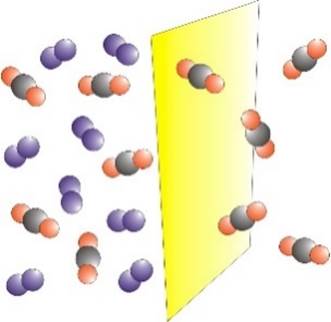
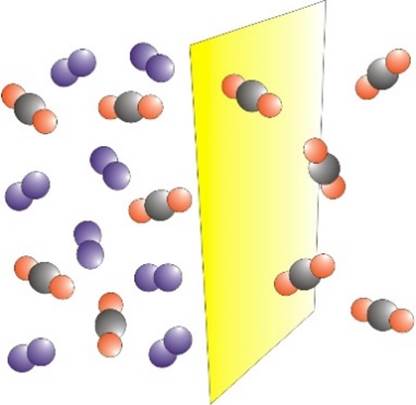
GAS SEPARATION Membranes may be used to
separate a mixture of gases. The process is driven by a difference in
pressure across the membrane. One of the first large-scale
applications of membrane gas separation was for the recovery of hydrogen in
the production of ammonia. |
|
|
CARBON DIOXIDE CAPTURE In order to reduce greenhouse
gas emissions, CO2 may be separated from flue gases or other
sources, to be utilised in useful products or stored in geological
formations. The most highly developed
technology for CO2 capture from flue gases is amine-based
absorption. Membrane systems, which are relatively simple and compact, are
being developed for this application. |
|
|
GAS TREATMENT Membrane systems may be used
to remove CO2 and other impurities from natural gas or biogas. |
AIR
SEPARATION Air is 78% N2 and 21% O2 by
volume, with small amounts of other gases. Air separation membranes are used to
create a nitrogen enriched inert gas, to reduce the risk of explosion, for
example in aircraft fuel tanks. Air separation membranes may also be used to
generate an oxygen enriched gas for industrial or other processes. |
|
FUEL CELLS A
fuel cell converts the chemical energy of a fuel into electrical energy. A
fuel cell continues to produce electricity as long as the fuel is supplied. In
a fuel cell an electrolyte is sandwiched between two electrodes. A fuel (e.g.
H2) is fed to the anode and an oxidant (e.g. O2 in air)
is fed to the cathode. |
PROTON-EXCHANGE MEMBRANE A proton-exchange membrane (PEM),
also called polymer electrolyte membrane (PEM), conducts protons but acts as
a barrier to other species and is an electronic insulator. A PEM may be used as an
electrolyte in a hydrogen-oxygen fuel cell. A commercial polymer commonly
used as a PEM is Nafion. Nafion is a copolymer of tetrafluoroethylene and a
perfluoro(vinyl ether) unit modified to give a sulfonic acid group. |
|
|
|