|
|
REVERSE OSMOSIS |
MENU
|
REVERSE OSMOSIS |
||
|
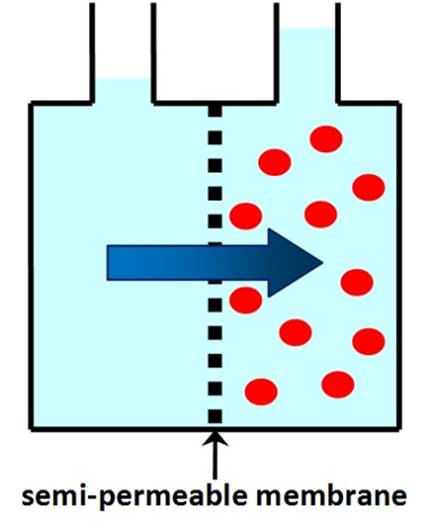
A SEMI-PERMEABLE MEMBRANE allows
solvent molecules to pass through, but retains solute molecules or ions. Normal (forward) OSMOSIS is the
spontaneous movement of solvent through a semi-permeable membrane from an
area of low solute concentration to an area of high solute concentration. |
|
|
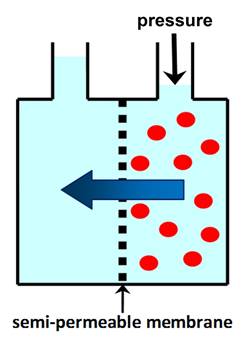
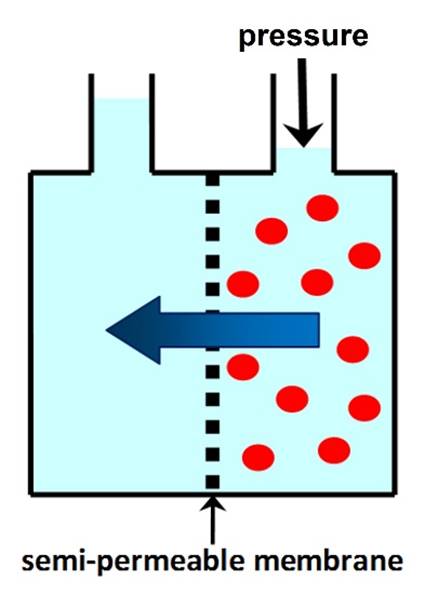
OSMOTIC PRESSURE is the minimum pressure that needs to be
applied to the solution to prevent the flow of solvent through a semi-permeable membrane. In REVERSE OSMOSIS (RO)
applied pressure is used to overcome osmotic pressure and force solvent
through a semi-permeable membrane that retains the solute. |
|
|
DESALINATION Reverse osmosis (RO) is widely
used for the desalination of seawater. You
can find data on membranes for water purification and desalination in the
Open Membrane Database. |
|
|
|
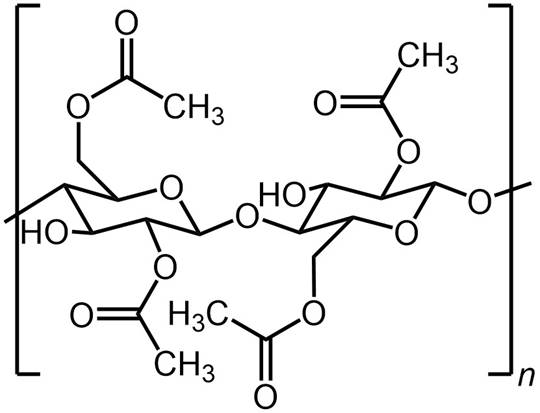
CELLULOSE ACETATE MEMBRANES The first commercial RO membranes were based on
cellulose acetate. Cellulose acetate membranes were developed in the 1950s that showed high salt
rejection but low flux. In 1963, Sidney Loeb and Srinivasa Sourirajan published
a method for fabricating high flux desalination membranes from cellulose
acetate. |
|
|
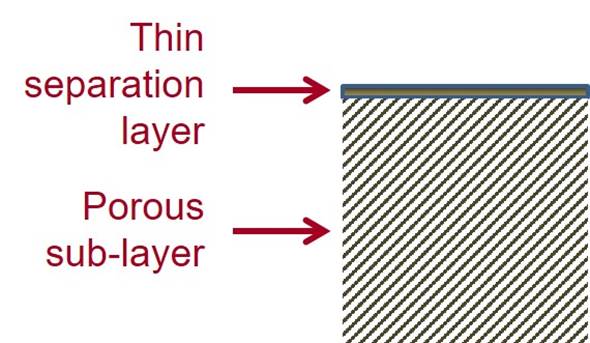
Cellulose acetate RO membranes are integrally skinned asymmetric membranes with a thin separation
layer and a highly porous sub-layer. The thin separation layer enables high flux to be achieved and the porous sub-layer provides mechanical
support. Integrally skinned means that the skin and the sub-layer are
formed from the same material, with the structure arising from the way
it is fabricated. |
|
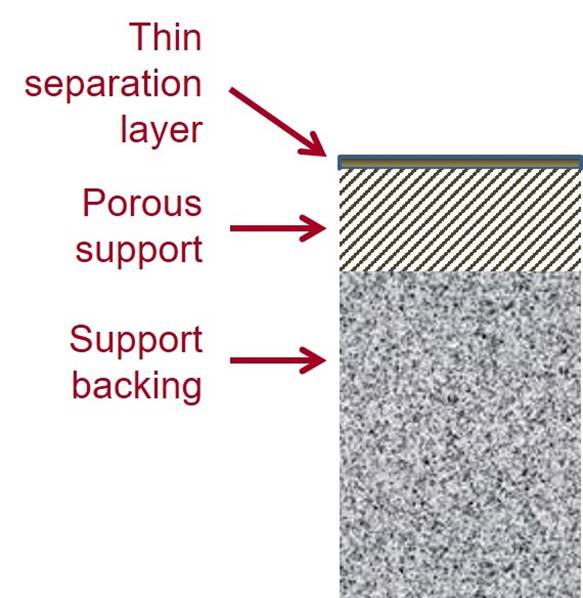
AROMATIC POLYAMIDE MEMBRANES Many current RO membranes are thin film composite (TFC)
membranes, which have a thin separation layer of one material on a porous
support made of a different material. Often they have a separation layer of an AROMATIC POLYAMIDE formed by interfacial polymerization on a porous polysulfone support with a nonwoven
polyester backing. |
|
|
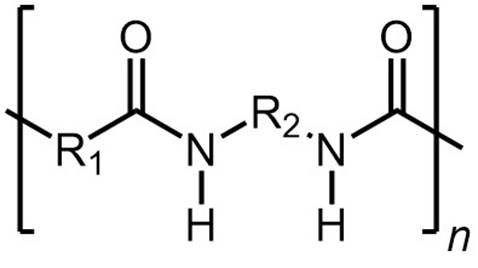
Synthetic polyamides are prepared by step-growth (condensation) polymerization
of a
monomer with amine groups and
a monomer with carboxylic acid or
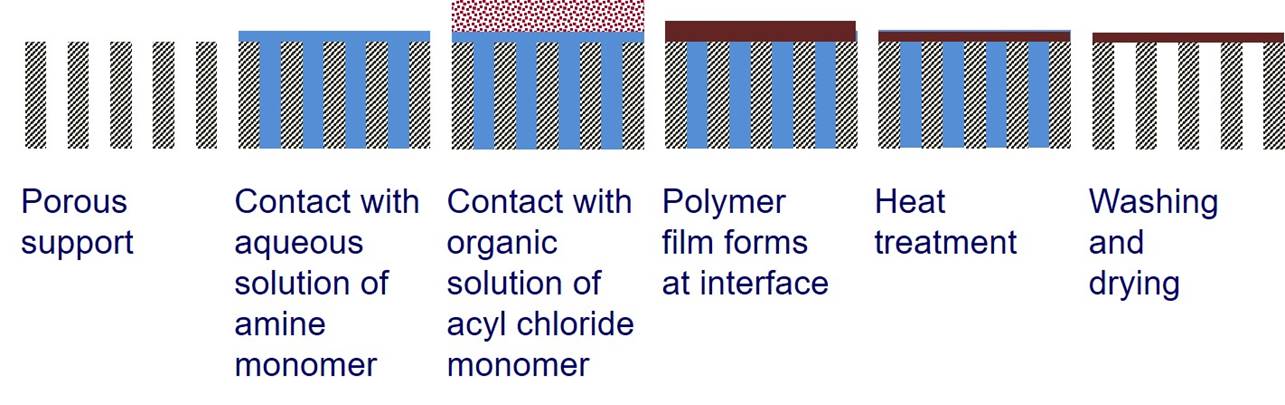
acyl halide groups. Interfacial polymerization is a type of step-growth polymerization where polymerization
occurs at the interface between two immiscible liquids. Usually one monomer is in an aqueous phase and the other
monomer is in an organic phase. Interfacial polymerization can
be used to create a thin polymer film at the surface of a porous
support. |
|
|
|
|
|
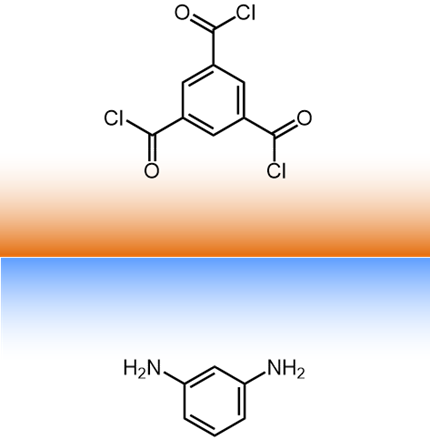
For a typical interfacial polymerization, 1,3,5-benzenetricarbonyl
trichloride (triemsyl
chloride) is in the organic phase and benzene-1,3-diamene (m-phenyldiamine)
is in the aqueous phase. |
|
|
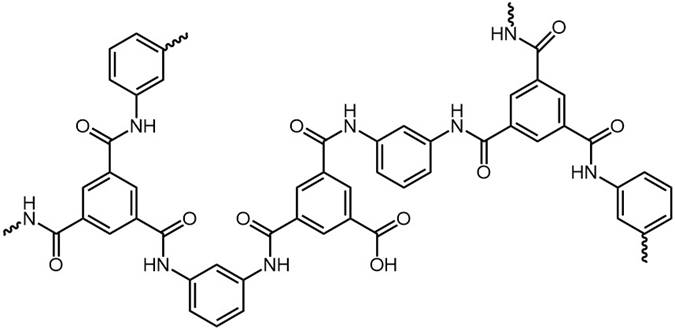
A crosslinked polymer film is obtained. Some unreacted acyl chloride groups may subsequently be hydrolysed to carboxylic acid. |
|
|
|
|