|
|
GAS SEPARATION |
MENU
|
GAS SEPARATION |
||
|
GAS
SEPARATION APPLICATIONS Gas separation membranes are used for applications such as: Air separation for generating nitrogen. Hydrogen recovery in ammonia production. CO2 removal from natural gas. |
INERT
GAS GENERATION Many aircraft have an on-board inert gas generating
system. A membrane module is used to
generate a nitrogen-rich inert gas from air. The inert gas is fed into the
fuel tank to prevent ignition of fuel vapours. |
|
MEMBRANE MATERIALS A membrane material must offer: SELECTIVITY so there is a separation. PERMEABILITY so the required membrane area is not too large. PROCESSABILITY so membranes can be fabricated economically. GOOD MECHANICAL PROPERITES so the membrane doesn’t fall apart. CHEMICAL AND THERMAL STABILITY so the mebrane
survives conditions of use. RESISTANCE TO AGEING, PLASTICIZATION AND FOULING so the membrane
maintains its performance over time. |
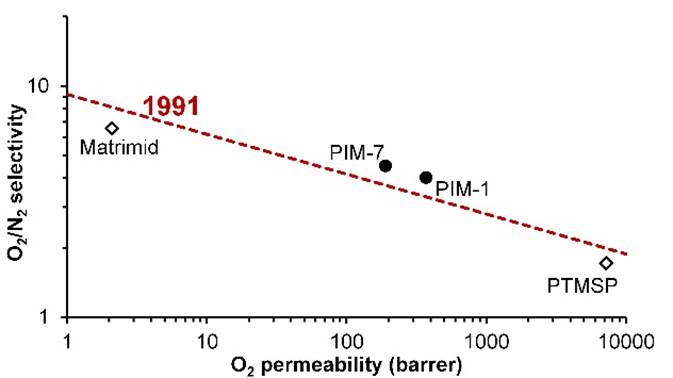
ROBESON PLOT Efforts to increase
selectivity often lead to a loss of permeability and vice versa. There
is a trade-off between these two properties. For gas separation membranes,
the trade-off between selectivity and permeability can be represented on a
double logarithmic plot. In 1991 Lloyd Robeson drew the
upper bound of performance that could then be achieved with polymer membranes
for various gas pairs. |
|
O2/N2
ROBESON PLOT Conventional membrane
polymers, such as the polyimide Matrimid, have low
permeability. The substituted polyacetylene
poly[1-(trimethylsilyl)-1-propyne] (PTMSP) has very high permeability, but
low selectivity. In 2005, two polymers of
intrinsic microporosity (PIMs) were shown to surpass the upper bound of
performance. |
L.M. Robeson, J.
Membr. Sci., 1991, 62, 165. P.M. Budd et al., J.
Membr. Sci., 2005, 252, 263. |
|
L.M. Robeson, J. Membr.
Sci., 2008, 320, 390. |
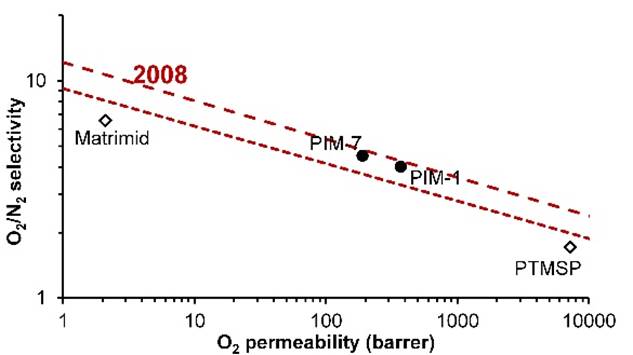
In 2008, Robeson revised the
upper bound. |
|
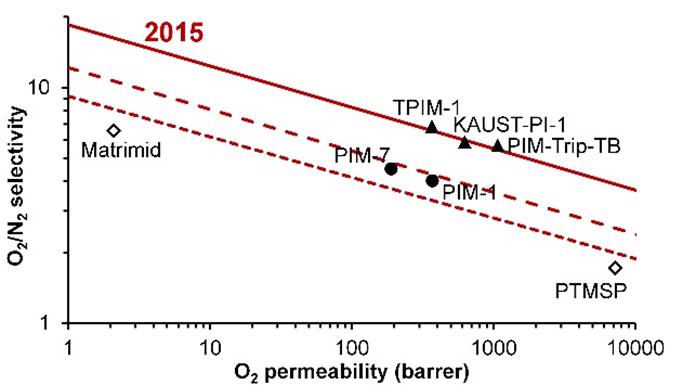
Further developments in PIMs led to a new upper bound being
proposed in 2015. |
R. Swaidan
et al., ACS Macro Lett., 2015, 4,
947. |
|
B. Comesana-Gandara
et al., Energy Environm. Sci., 2019, 12, 2733-2740. |
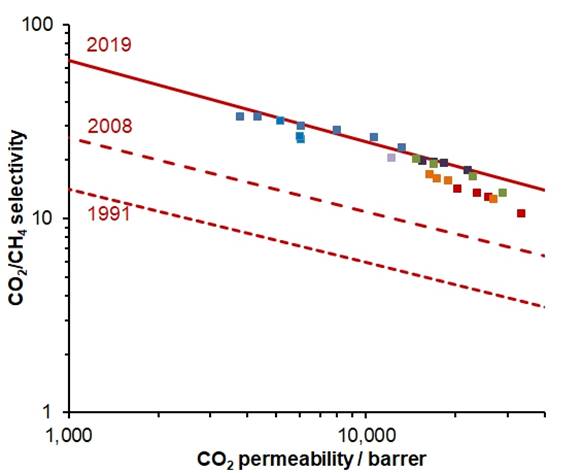
CO2/CH4
ROBESON PLOT In 2019, new upper bounds were proposed for CO2 separations, based on
ageing data for membranes of benzotriptycene-based
PIMs. |
|
You can find gas permeation data for many membrane materials in
a database maintained by CSIRO. |
|
|
Machine-learning can be used to predict gas permeability and other polymer
properties. |
|
|
Gas separation membranes are often based
on glassy polymers. High free volume, glassy polymers such as PIMs offer the
prospect of very permeable membranes, but for commercial application the
issue physical ageing, which leads to a loss of permeability over time, needs
to be addressed. |
gas permeation properties,
physical ageing, and Its mitigation in high free volume glassy polymers |
|
To improve performance, a filler may be added
to a polymer, giving a mixed matrix membrane (MMM), or two different polymers
may be blended together. |
seeking synergy in
membranes: blends and mixtures with polymers of intrinsic microporosity |
|
|
|