The Mace Lab
| Home | Publications | Protocols | Links | Contact Us |
 |
Regulation of adult stem cell recruitment, engraftment, and differentiation during tissue repair and regeneration Overview Tissue repair and regeneration is an ancient response of all metazoans to physical damage that requires coordinated cell movements and changes in gene expression. My lab is particularly interested in the regulation of transcription in response to injury, and the underlying molecular mechanisms that promote wound repair and regeneration, as well as limit it. Current studies are focused on skin repair and regeneration, specifically adult stem cell recruitment and angiogenesis, using the mouse as a model system. Adult stem cell recruitment to injured tissue
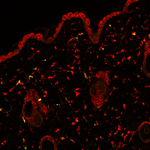
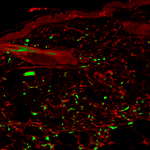
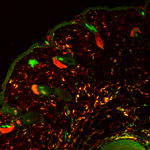
Adult stem cells reside in a variety of niches, including the bone marrow. Bone marrow-derived cells (BMDCs) contain a subpopulation of multipotent stem cells that can migrate to peripheral tissues and, once there, differentiate and contribute to the maintenance of that tissue. BMDCs also appear to play an extensive role in repair and regeneration. For example, in skin, a small number of BMDCs routinely migrate to the dermis and contribute to many resident cell populations. This process is dramatically 'ramped up' in response to injury, and then returns to normal as wound healing resolves and homeostasis is achieved.
Current projects include:
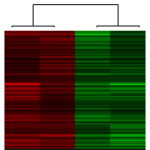
Transcriptional regulation in response to injury Several Hox transcription factors are upregulated in response to injury. These transcription factors, in turn, regulate downstream target genes which influence cell function and cell behaviour. Microarray analysis of Hoxa3 target genes during wound repair in vivo identified a number of genes regulated by Hoxa3 during tissue repair and regeneration. In particular, we are interested in a subset of genes which may influence BMDC trafficking to injured tissue. Current projects include:
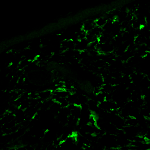
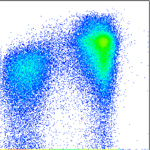
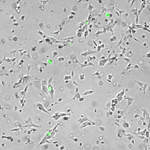
Regulation of inflammatory cells in injured tissue Using GFP bone marrow chimeras, we are analyzing the behaviour of inflammatory cells in different wound environments, such as chronic wounds and acute (normal) wounds. Sustained expression of Hoxa3 during tissue repair and regeneration reduces inflammation and accelerates healing, however, it is unclear if this is direct or indirect (whether Hoxa3 function is required in the wound resident cells, the inflammatory cells, or both). We would like to better understand the regulation of inflammatory cell gene expression in response to injury. Current projects include:
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
| This page was last updated by KM on 12/09/07 |