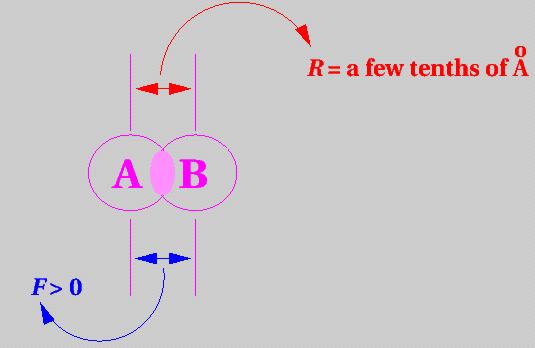
At close range, when the electron density around one atom or molecule is large in the same region of space as the electron density around the other atom or molecule (i.e. when filled orbitals overlap) charge repulsion dominates and the two atoms or molecules repel each other.
The force between them, F, is positive (repulsive).
[ Next Page ]
[ Home ]
[ Previous Page ]

