Methane
|
|
|
|
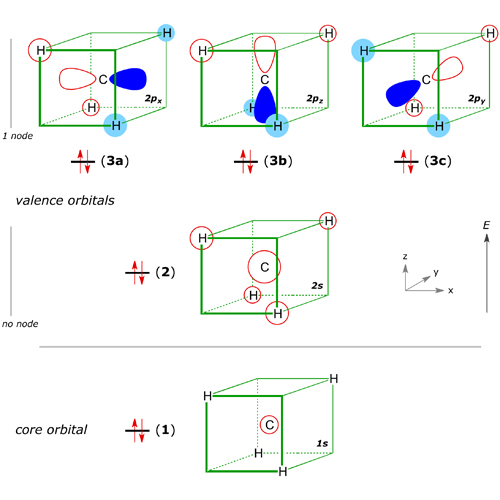
The occupied valence-shell MOs (2) and (3) are lower in energy than the non-bonding level (0 eV). MO(2) has an even distribution over the molecule, with the two electrons shared over the C and all four H atoms. The three MOs (3a), (3b) and (3c) are degenerate, differing only in their spatial orientation. Each incorporates a phase change (node), represented by the colour change from blue to red, with an associated decrease in the net bonding compared to MO(2), which has no node. Each of the three MOs (3) has in-phase electron density between the C atom and two of the H atoms. The bonding MOs are all delocalised, with high electron density between C and H. We can get the same picture by the mathematical combination of C(sp3)–H(1s) valence bonds:
|
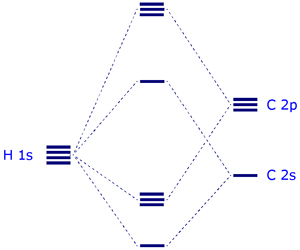
The energy level diagram below shows how the four H (1s) orbitals and the four C orbitals (2s and three 2p) mix to generate four bonding and four antibonding molecular orbitals. N.B. The carbon 1s core orbital MO(1) is not shown on this diagram.
|