Van Der Waals Forces
There are two principal van der Waals forces. The most important force at short range is the repulsion between electrons in the filled orbitals of atoms on neighbouring molecules.
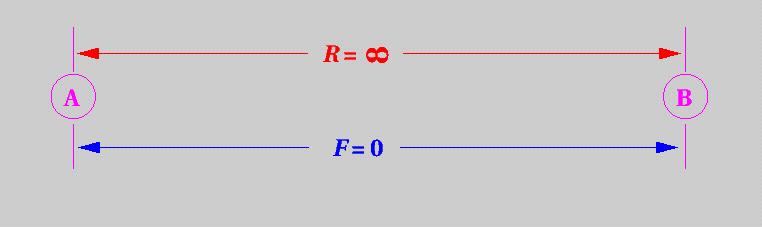
At very large distances, R, (see below) two atoms or molecules behave toward each other as neutral species and neither repel nor attract one another. The force between them, F, is zero.
[ Next Page ]
[ Home ]
[ Previous Page ]

