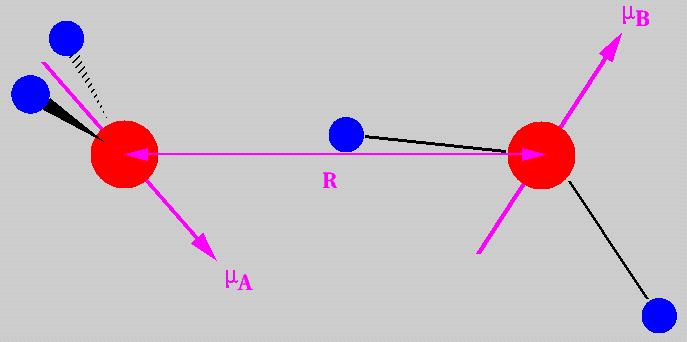
The interaction energy, E, between two permanent dipoles mA and mB with their centres separated by R, varies according to the orientation of the dipole moments.

The interaction energy, E, between two permanent dipoles mA and mB with their centres separated by R, varies according to the orientation of the dipole moments.