
Thermoreversible gelation of stereoregular poly(methyl methacrylate)s
The
purpose of this work was to provide a fundamental understanding of
polymer-solvent interactions in the context of helix formations.
Polymer-solvent
interactions have received considerable attention as these
interactions, whether
desirable or undesirable, are present in most of the applications
involving the
use of polymers.
Of particular interest is the influence of the solvent on polymer conformation, which can strongly influence the resulting properties of the material. For instance, bromobenzene can induce, via polymer-solvent interaction, crystallisation of syndiotactic PMMA but, when the solvent is removed, the sample returns to the amorphous state [H. Kusuyama et al., Polym. Commun., 1983, 24, 119]. It has been noticed that many synthetic [polystyrene, PMMA, poly(vinyl pyridine)] or biologic [agarose, gelatine] polymers exhibit temperature induced conformational transitions in dilute or semi-dilute solutions, which are usually solvent dependent [J.-M. Guenet Thermoreversible Gelation of Polymers and Biopolymers; Acad. Press, London (1992).]
A remarkable feature of stereoregular PMMAs is their ability to form helicoidal structures and especially double helix structures, which are often encountered in biological systems but more rarely in synthetic polymers. These double stranded helices can be formed from the bulk, as in the case of isotactic PMMA, for which a symmetric 101 double helix was proposed for the chain conformation in crystalline samples [ H. Kusanagi et al., Macromolecules, 9, 531 (1976)]. They can also be solvent-induced as in syndiotactic PMMA, for which we suggested the presence of an asymmetric double helix in syndiotactic PMMA gels.
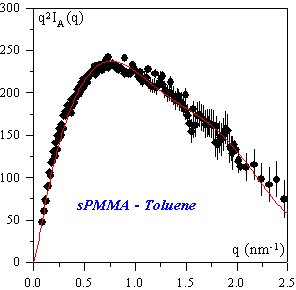
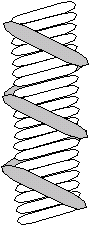
Kratky representation of the intensity scattered by a 35% (v/v) syndiotactic PMMA gel containing 6% (v/v) deuterated chains in bromobenzene at 25 ºC (left). The solid line represent to the best fit obtained by using a double helix model (right).
A unique feature of stereoregular PMMAs is the existence of the so-called stereocomplex that is obtained by mixing isotactic and syndiotactic chains in a ratio 2:1 [T.G. Fox et al., J. Am. Chem. Soc., 80, 1768 (1958)]. This ratio was explained by the existence of an asymmetric double helix where the inner helix is made by an isotactic chain (91 helix) around which a syndiotactic chain wraps adopting a 181 conformation [E. Schomaker et al., Macromolecules, 22, 3337 (1989)]. Interestingly, syndiotactic PMMA seems to be able to adopt several helicoidal conformations.
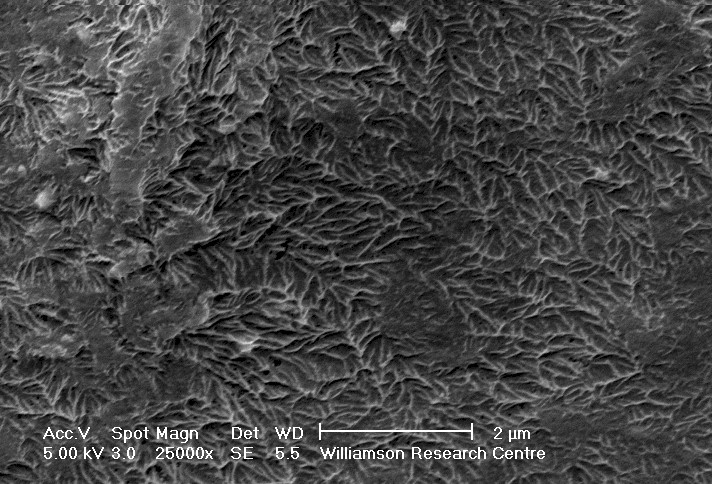
CryoSEM micrograph of a 12% (v/v) stereocomplex gel prepared in toluene.
Another particularity of stereoregular PMMAs is their ability to form thermoreversible or "physical" gels in a large variety of solvents, yet not necessarily in the same solvent. For instance, while strong aggregation occurs in toluene for highly syndiotactic PMMA, this aggregation seems to be low for isotactic PMMA. The stereocomplex also has the ability to form gels in some solvents and particularly in bromobenzene, for which the presence of a fibrillar network whose junctions consist of the aggregation of three double helices was proposed [N. Fazel et al., Macromolecules, 27, 3836 (1994)]. The same kind of network model was proposed for syndiotactic PMMA and for carrageenan gels [J.-M. Guenet et al., Trends in Macromol. Res., 1, 345 (1994)]. Interestingly, this last biopolymer also forms double helical structures.
Publications:
Gelation dynamics and mechanism(s) in stereoregular poly(methyl methacrylate)s; A. Saiani; Macromolecular Symposia, 222, 37-47 (2005)
Nanostructure and helicity in syndiotactic poly[methyl methacrylate] thermoreversible gels; A. Saiani, J.-M. Guenet; Macromolecules, 32, 657-663 (1999)
Phase behaviour and polymer/solvent interactions in thermoreversible gels of syndiotactic poly[methyl methacrylate]; A. Saiani, J. Spevacek, J.-M. Guenet; Macromolecules, 31, 703-710 (1998)
Temperature-concentration phase diagrams in the study of thermoreversible gelation; D. López, A. Saiani, J.-M. Guenet, Journal of Thermal Analysis and Calorimetry, 51, 841-851 (1998)
On the helical form in syndiotactic poly[methyl methacrylate] thermoreversible gels as revealed by small-angle neutron scattering; A. Saiani, J.-M. Guenet; Macromolecules, 30, 966-972 (1997)
Thermoreversible gelation of isotactic poly[methyl methacrylate] in butylacetate : a DSC study; J. Spevacek, A. Saiani, J.-M. Guenet; Macromolecular Rapid Communications, 17, 389-395 (1996)
________________________________________
Home Research Projects Publications Group members Links Contact us