
Self-Assembly and Gelation of Fmoc-Dipeptide
Over the last decade significant efforts have been made to develop a new generation of biomaterials based on the self-assembly of peptides and their derivatives. By exploiting the spontaneous or induced molecular arrangement of peptides and their derivatives in aqueous solutions, nanostructured hydrogels can be formed under specific conditions. These highly hydrated scaffolds have potential applications in tissue engineering, 3D cell culture, and templating. Such peptide based biomaterials exploit known biological architectures like beta-sheet. A relatively new class of hydrogel scaffolds formed from the self-assembly of much shorter peptides modified with aromatic ligands has recently been developed. The aromatic moieties play a key role in the self-assembly process through pi-pi interactions, while the peptide components are stabilised via hydrogen bonding.
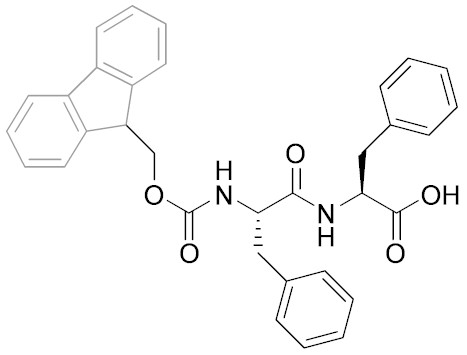
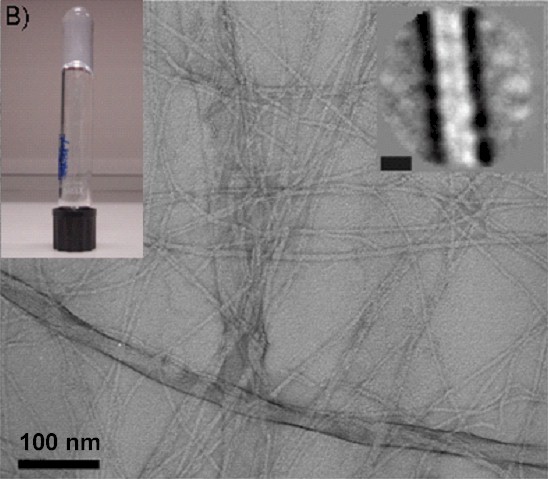
Left: chemical structure of Fmoc-diphenylalanine (Fmoc-FF). Right: TEM micrographs of Fmoc-FF samples at pH 9 and 10 mmol L-1 (right hand side insert: projection average of the fibres, scale bar represents 6 nm; left hand side inset: photograph of the sample showing its macroscopic aspect)
The main focus of this project is to characterise the self-assembling behaviour of a family of dipeptides possessing an N-terminal fluorenyl-9-methoxycarbonyl (Fmoc) group. The overall aim being to establish design rules allowing the preparation of biomaterials, in particular hydrogels, with tailored properties for cell culture and tissue engineering applciations.
The self-assembly and gelation properties of these peptide derivatives are investigated as a function the media pH using transmission electron microscopy (TEM), potentiometry, wide angle X-ray scattering (WAXS), infrared spectroscopy (ATR-FTIR) and dynamic rheology.
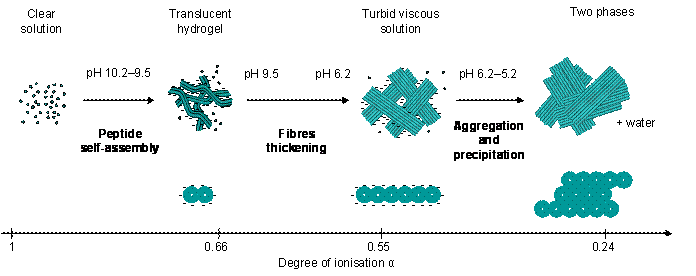
Proposed self-assembly mechanism of Fmoc-FF from high to low pH above the critical gelation concentration as a function of the peptide degree of ionisation.
Publications:
Self-assembly and gelation properties of glycine / leucine Fmoc-dipeptides C. Tang, R.V. Ulijn, A. Saiani; European Physical Journal E - Soft Matter and Biological Physics, 36:111 (2013)
Effect of glycine substitution on Fmoc-diphenylalanine self-assembly and gelation properties; C. Tang, R.V. Ulijn; A. Saiani; Langmuir, 27, 14438-14449 (2011)
Fmoc-diphenylalanine self-assembly mechanism induces apparent pKa shifts C. Tang, A.M. Smith, R.F. Collins, R.V. Ulijn, A. Saiani Langmuir, 25, 9447–9453 (2009)
Introducing chemical functionality in Fmoc-peptide gels for cell culture V. Jayawarna, S.M. Richardson, A. Hirst, N.W. Hodson, A. Saiani, J.E. Gough, R.V. Ulijn Acta Biomaterialia, 5, 934-943 (2009)
Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on p -p interlocked b-sheets A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani, R. V. Ulijn, Advanced Materials, 20, 37-41 (2008)
Design of nanostructured hydrogels for 3D cell culture through self assembly of Fmoc-dipeptides; V. Jayawarna, M. Ali, A.F. Miller, A. Saiani, J.E. Gough, R.V. Ulijn; Advanced Materials, 18, 611-614 (2006)
________________________________________