
Protein Hydrogel Scaffolds for 3D Tissue Engineering
Hydrogels have many important applications in the biomaterials field, such as superabsorption, drug delivery and tissue engineering. Traditionally, hydrogels are fabricated from high molecular weight, synthetic amphiphilic polymers that are cross-linked through either physical entanglements or covalent bonds. As an alternative, proteins have attracted considerable attention in the development of novel biomaterials as they self-assemble into ordered supramolecular architectures on the meso- to macroscopic length scales and are potentially biocompatibile and biodegradable. In particular, significant interest has focused on the b-sheet motif due to its important role in the formation of protein fibrils. Such fibrils have recently been shown to further self-organize into a three-dimensional network, i.e. a protein hydrogel.
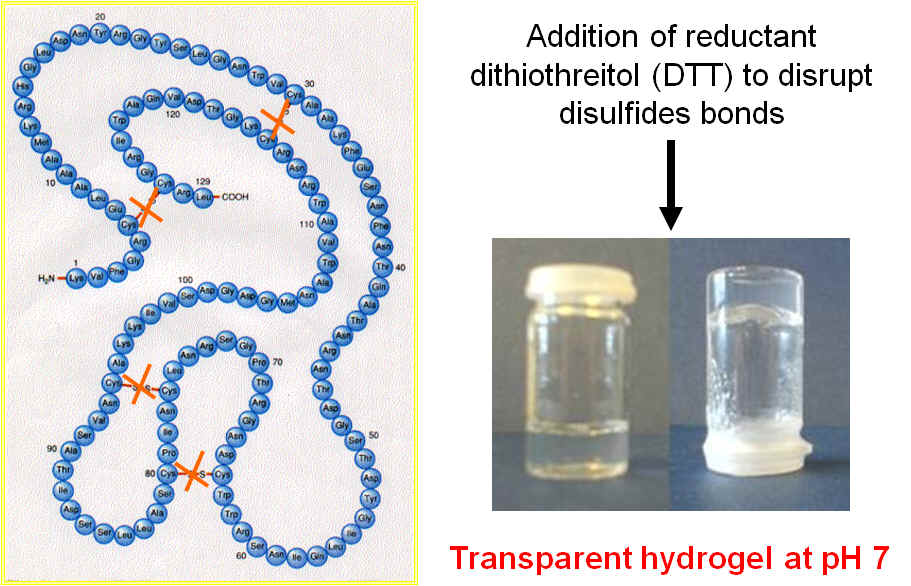
Left: structure of HEWL; Right: Photograph of hydrogels obtained at pH 7 in the presence of DTT.
Here we investigate the gelation properties of hen egg white lysozyme (HEWL). The overall aim is to establish design rules allowing the preparation of hydrogels in cell culture conditions with tailored properties. The work to date has focussed on the use of dithiothreitol (DTT) to disrupt the disulfide bridges in the protein to promote its fibrillisation and the formation of hydrogels under mild conditions i.e.: pH 7.
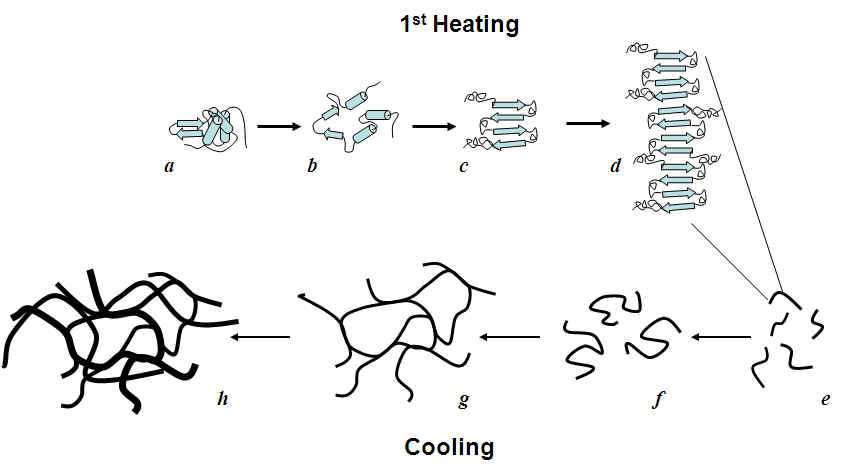
Proposed self-assembly mechanism of HEWL in presence of DTT.
The self-assembly and gelation properties of HEWL are investigated using a combination of techniques including: transmission electron microscopy (TEM), small angle neutron scattering (SANS), infrared spectroscopy (ATR-FTIR) and dynamic rheology. This has allowed us to understand the gelation pathway of HEWL in the presence of DTT. The gels formed were then shown to sustain human dermal fibroblast (hDF) culture.
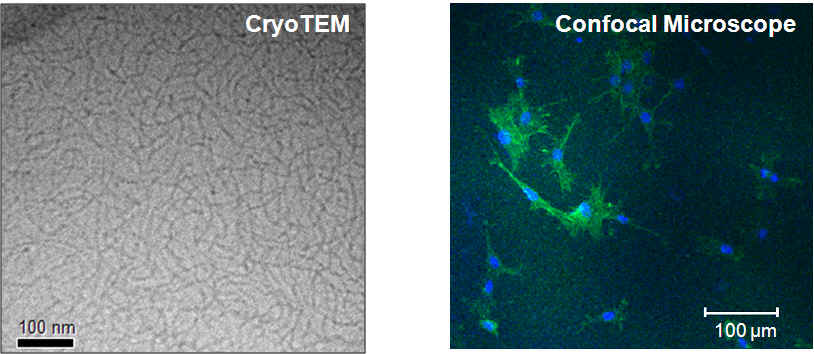
Left: CryoTEM micrograph of HEWL fibrillar network formed in the presence of DTT; Right: Confocal micrograph of hDF cultured in the hydrogels for 7 days.
Publications:
Protein fibrillar hydrogels for 3-dimensional tissue engineering H. Yan, A. Nykanen, J. Ruokolainen, D. Farrar and A.F. Miller Research Letters in Nanotechnology, 2009, 614301 (2009)
Thermoreversible protein fibrillar hydrogels as cell scaffolds; H. Yan, A. Nykanen, J. Ruokolainen, A. Saiani, A.F. Miller; Faraday Discussions, 139, 71–84 (2008)
Thermoreversible lysozyme hydrogels: Properties and an insight into the gelation pathway; H. Yan, H. Frielinghaus, A. Nykanen, J. Ruokolainen,, J.E. Gough, A. Saiani, A.F. Miller; Soft Matter, 4, 1313-1325 (2008)
Gelation of a model globular protein; H. Yan, A. Saiani, A.F. Miller; Macromolecular Symposia, 251, 112-117 (2007)
Thermo-reversible protein hydrogel as cell scaffold; H. Yan, A. Saiani, J.E. Gough, A.F. Miller; Biomacromolecules, 7, 2776-2782 (2006)
________________________________________
Home Research Projects Publications Group members Links Contact us